Solution 1 Answer: Justices practice judicial restraint when they make narrow decisions that only relate to a specific case. Explanation: Judicial restraint is a principle of separation of powers that must be taken into account in judicial decision-making.
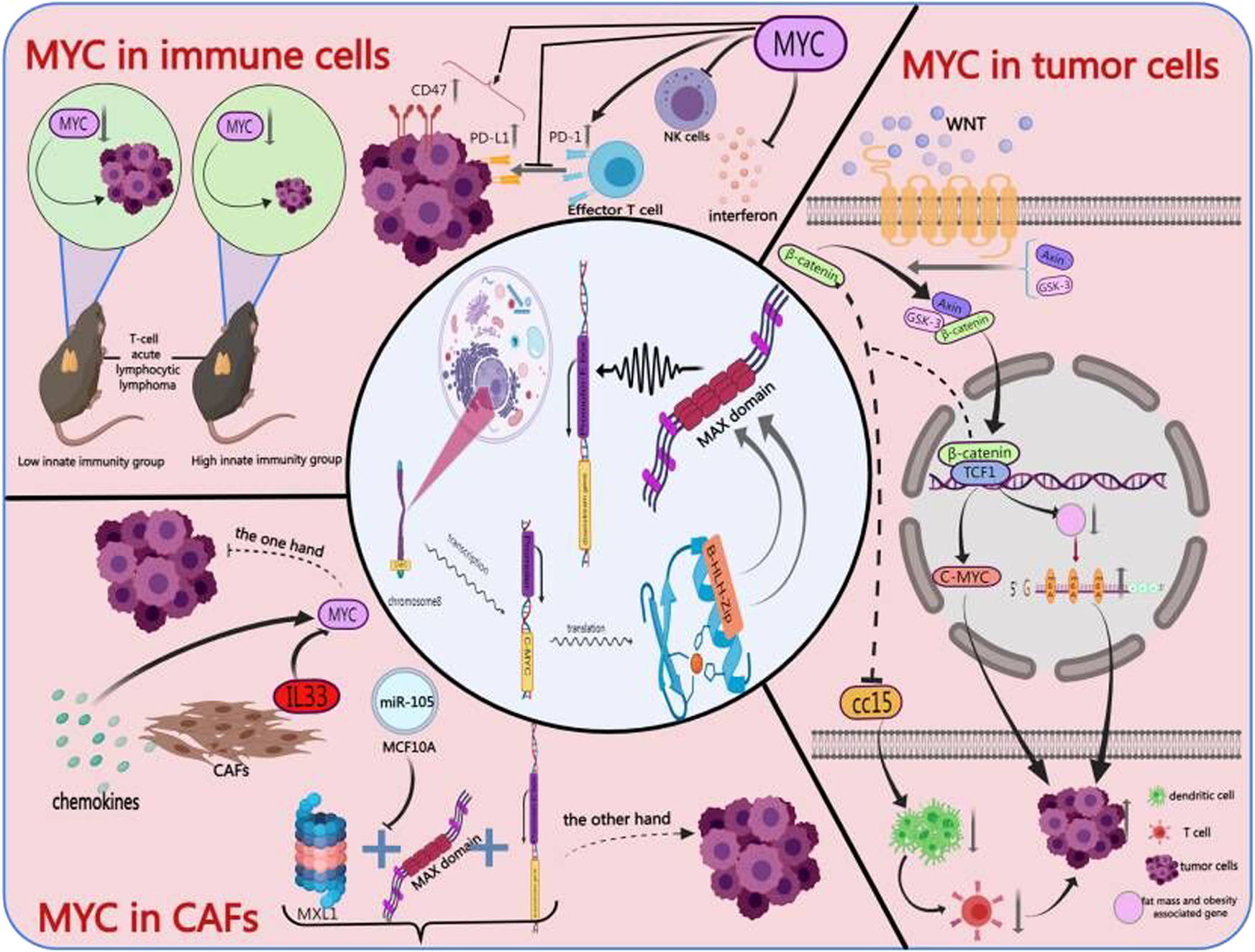
The effects of MYC on tumor immunity and immunotherapy | Cell Death Discovery
Step 4: Assess whether non-approvable groups are identifiable without added screening. Simply in the course of conducting clinical trials, investigators sometimes identify individuals as pregnant, prisoners or minors. When clinical trials are conducted in prisons, it typically is obvious which individuals are prisoners.

Source Image: cell.com
Download Image
good clinical practice, human subject protection, informed consent, institutional review board, sponsor, trial, study, investigator, monitor, FDA, IRB
Source Image: nature.com
Download Image
Literature review in research | PPT
May 30, 2022It also found that the survey participants saw many benefits to clinical trial participation beyond clinical benefit, including hope for a better future, the opportunity to help others, and positive relationships with study teams. This research provides new perspective on patient experiences in SMA clinical trials.

Source Image: neurology.org
Download Image
A Subject In A Clinical Research Trial Experiences A Serious
May 30, 2022It also found that the survey participants saw many benefits to clinical trial participation beyond clinical benefit, including hope for a better future, the opportunity to help others, and positive relationships with study teams. This research provides new perspective on patient experiences in SMA clinical trials.
Clario: Keeping an eye on clinical trial technology is much easier with the blog from software vendor Clario. They often discuss the latest best practices in the space and cover emerging innovations that may shape the future of medical research. Clinical Leader: Clinical Leader is a great resource for news regarding study design, clinical trial
Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis | Neurology
A subject in a clinical research trial experiences a serious, unanticipated adverse drug experience. How should the investigator proceed, with respect to the IRB, after the discovery of the adverse event occurrence? Click the card to flip 👆
Nearly 1 in 5 Adults May Have Misophonia, Experiencing Significant Negative Responses to Sounds – Neuroscience News

Source Image: neurosciencenews.com
Download Image
Nader Ale Ebrahim – Research Visibility and Impact Center (RVnIC) | LinkedIn
A subject in a clinical research trial experiences a serious, unanticipated adverse drug experience. How should the investigator proceed, with respect to the IRB, after the discovery of the adverse event occurrence? Click the card to flip 👆

Source Image: ir.linkedin.com
Download Image
The effects of MYC on tumor immunity and immunotherapy | Cell Death Discovery
good clinical practice, human subject protection, informed consent, institutional review board, sponsor, trial, study, investigator, monitor, FDA, IRB
Source Image: nature.com
Download Image
Literature review in research | PPT
Solution 1 Answer: Justices practice judicial restraint when they make narrow decisions that only relate to a specific case. Explanation: Judicial restraint is a principle of separation of powers that must be taken into account in judicial decision-making.

Source Image: slideshare.net
Download Image
Best Clinical Trials Courses & Certificates Online [2024] | Coursera
Feb 16, 2023Planning clinical trial/research. The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
Source Image: coursera.org
Download Image
Experimental research design | PPT
May 30, 2022It also found that the survey participants saw many benefits to clinical trial participation beyond clinical benefit, including hope for a better future, the opportunity to help others, and positive relationships with study teams. This research provides new perspective on patient experiences in SMA clinical trials.

Source Image: slideshare.net
Download Image
The Puerto Rico Pill Trials | American Experience | Official Site | PBS
Clario: Keeping an eye on clinical trial technology is much easier with the blog from software vendor Clario. They often discuss the latest best practices in the space and cover emerging innovations that may shape the future of medical research. Clinical Leader: Clinical Leader is a great resource for news regarding study design, clinical trial

Source Image: pbs.org
Download Image
Nader Ale Ebrahim – Research Visibility and Impact Center (RVnIC) | LinkedIn
The Puerto Rico Pill Trials | American Experience | Official Site | PBS
Step 4: Assess whether non-approvable groups are identifiable without added screening. Simply in the course of conducting clinical trials, investigators sometimes identify individuals as pregnant, prisoners or minors. When clinical trials are conducted in prisons, it typically is obvious which individuals are prisoners.
Literature review in research | PPT Experimental research design | PPT
Feb 16, 2023Planning clinical trial/research. The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.