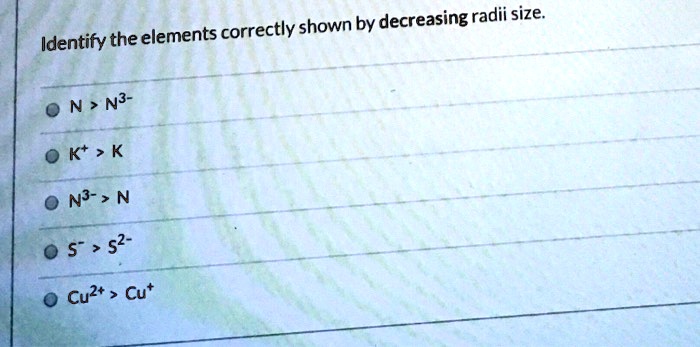
Identify the elements correctly shown by decreasing radii size. A) N3-> N B) K+> K C) N > N3- D) Cu2+> Cu+ E) S-> S2-Identify the elements correctly shown by decreasing radii size. A) N3-> N B) K+> K C) N > N3- D) Cu2+> Cu+ E) S-> S2- … Chemistry. Study Set. Chemistry Study Set 2. Quiz. Quiz 8: Periodic Properties of the Elements. Solved
Arrange the following elements in order of increasing atomic radii. a. S b. Na c. Mg d. Si e. Cl | Homework.Study.com
Science Chemistry Identify the elements correctly shown by decreasing radii size. ON> N3- Cu2+ > Cu* N3- >N S > s2- O K* > K Identify the elements correctly shown by decreasing radii size. ON> N3- Cu2+ > Cu* N3- >N S > s2- O K* > K BUY Chemistry: Matter and Change 1st Edition ISBN: 9780078746376
Source Image: quora.com
Download Image
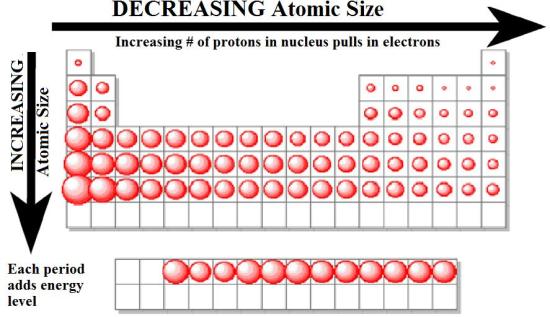
Figure 3.2.5 The Atomic Radius of the Elements. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. The increase in atomic size going down a column is also due to electron shielding, but the situation is more complex because the principal quantum number n is not constant.
 Download Image
Download ImageWhich arrangement is in the correct order of radius size? a) Mn > Mn2+ > Cs b) Li+ > Li > Ra c) P < P3– < As3– d) Cr < Cr3+ < Ca e) Al3+ > Al > Si | Socratic
Which of the following statements is/are CORRECT? For any element, the second ionization energy is larger than the first ionization energy. Ionization energy is a positive value for all elements. Ionization energy increases down a group of the periodic table. a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1, 2 and 3. Answer
 Download Image
Download ImageIdentify The Elements Correctly Shown By Decreasing Radii Size
Which of the following statements is/are CORRECT? For any element, the second ionization energy is larger than the first ionization energy. Ionization energy is a positive value for all elements. Ionization energy increases down a group of the periodic table. a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1, 2 and 3. Answer
Identify the elements correctly shown by decreasing radii size. A) N3-> N B) K+ > K CN > N3- D) Cu2+ > Cut E) S-> 32- TDTES Not the question you’re looking for? Post any question and get expert help quickly. Start learning Answer to Solved D) SP 12. Identify the elements correctly shown by | Chegg.com
SOLVED: Identify the elements correctly shown by decreasing radii size: N > N3- Kt > K N3- > N 5″ > 52- Cuzt Cut
Question: 1.) Identify the elements correctly shown by decreasing radii size. a.) S- > S2- b.) N3- > N c.) Cu2+ > Cu+ d.) K+ > K e.) N > N3 2.) Determine the final temperature of a gold nugget (mass = 376 g) that starts at 288 K and loses 4.85 kJ of heat to a snowbank when it is lost.
SOLVED: 3- Arrange the following elements in order of decreasing atomic radius: Cs, Sb, S, Pb, Se 4- Write the electronic configurations for each of the following ions: a. O2- b. Br-

Source Image: numerade.com
Download Image
Soil acidity fact sheet
Question: 1.) Identify the elements correctly shown by decreasing radii size. a.) S- > S2- b.) N3- > N c.) Cu2+ > Cu+ d.) K+ > K e.) N > N3 2.) Determine the final temperature of a gold nugget (mass = 376 g) that starts at 288 K and loses 4.85 kJ of heat to a snowbank when it is lost.

Source Image: yumpu.com
Download Image
Arrange the following elements in order of increasing atomic radii. a. S b. Na c. Mg d. Si e. Cl | Homework.Study.com
Figure 3.2.5 The Atomic Radius of the Elements. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. The increase in atomic size going down a column is also due to electron shielding, but the situation is more complex because the principal quantum number n is not constant.

Source Image: homework.study.com
Download Image
Which arrangement is in the correct order of radius size? a) Mn > Mn2+ > Cs b) Li+ > Li > Ra c) P < P3– < As3– d) Cr < Cr3+ < Ca e) Al3+ > Al > Si | Socratic
Identify the elements correctly shown by decreasing radii size. A) N3-> N B) K+> K C) N > N3- D) Cu2+> Cu+ E) S-> S2-Identify the elements correctly shown by decreasing radii size. A) N3-> N B) K+> K C) N > N3- D) Cu2+> Cu+ E) S-> S2- … Chemistry. Study Set. Chemistry Study Set 2. Quiz. Quiz 8: Periodic Properties of the Elements. Solved
 Download Image
Download ImageApplied Sciences | Free Full-Text | Numerical Investigation of Locating and Identifying Pipeline Reflectors Based on Guided-Wave Circumferential Scanning and Phase Characteristics
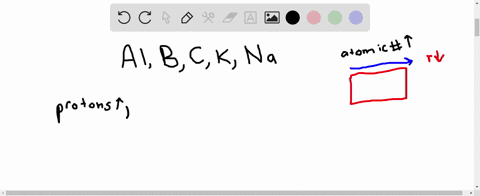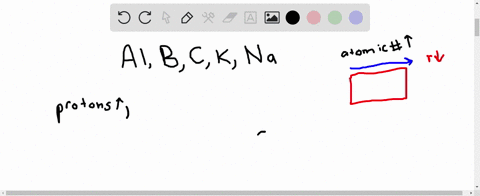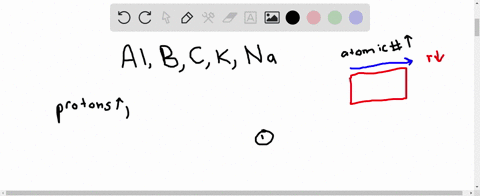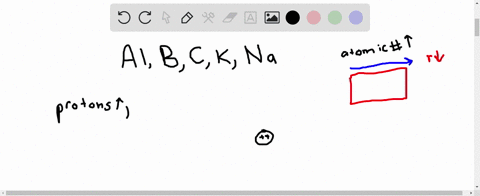
Jan 26, 2023Study with Quizlet and memorize flashcards containing terms like Give the ground state electron configuration for the ion of Ba., Identify the elements correctly shown by decreasing radii size., Place the following in order of increasing X-Se-X bond angle, where X represents the outer atoms in each molecule. SeO2 SeCl6 SeF2 and more.

Source Image: mdpi.com
Download Image
Periodic Table Trends- Atomic size, Melting & Boiling Point Trend
Which of the following statements is/are CORRECT? For any element, the second ionization energy is larger than the first ionization energy. Ionization energy is a positive value for all elements. Ionization energy increases down a group of the periodic table. a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1, 2 and 3. Answer

Source Image: byjus.com
Download Image
Generalized Reynolds equation for microscale lubrication between eccentric circular cylinders based on kinetic theory | Journal of Fluid Mechanics | Cambridge Core
Identify the elements correctly shown by decreasing radii size. A) N3-> N B) K+ > K CN > N3- D) Cu2+ > Cut E) S-> 32- TDTES Not the question you’re looking for? Post any question and get expert help quickly. Start learning Answer to Solved D) SP 12. Identify the elements correctly shown by | Chegg.com

Source Image: cambridge.org
Download Image
Soil acidity fact sheet
Generalized Reynolds equation for microscale lubrication between eccentric circular cylinders based on kinetic theory | Journal of Fluid Mechanics | Cambridge Core
Science Chemistry Identify the elements correctly shown by decreasing radii size. ON> N3- Cu2+ > Cu* N3- >N S > s2- O K* > K Identify the elements correctly shown by decreasing radii size. ON> N3- Cu2+ > Cu* N3- >N S > s2- O K* > K BUY Chemistry: Matter and Change 1st Edition ISBN: 9780078746376
Which arrangement is in the correct order of radius size? a) Mn > Mn2+ > Cs b) Li+ > Li > Ra c) P < P3– < As3– d) Cr < Cr3+ < Ca e) Al3+ > Al > Si | Socratic Periodic Table Trends- Atomic size, Melting & Boiling Point Trend
Jan 26, 2023Study with Quizlet and memorize flashcards containing terms like Give the ground state electron configuration for the ion of Ba., Identify the elements correctly shown by decreasing radii size., Place the following in order of increasing X-Se-X bond angle, where X represents the outer atoms in each molecule. SeO2 SeCl6 SeF2 and more.